

The Qualcomm Tricorder XPrize is a $10 million global competition developed to incentivize healthcare technology  innovation. The goal is to develop a hand-held device that would allow a consumer to access the state of their own health anytime, anywhere. The device will be able to capture key health metrics and diagnose a set of 15 diseases. These metrics could include blood pressure, respiratory rate and temperature. The device would ultimately collect large volumes of data from ongoing monitoring. There is a weight limit of 5 pounds, but other than that, there are no limits as to device physical appearance and functionality. A qualifying round will evaluate consumer experience so user satisfaction is important. Teams are also expected to ensure that consumer safety is held in the highest regard.
innovation. The goal is to develop a hand-held device that would allow a consumer to access the state of their own health anytime, anywhere. The device will be able to capture key health metrics and diagnose a set of 15 diseases. These metrics could include blood pressure, respiratory rate and temperature. The device would ultimately collect large volumes of data from ongoing monitoring. There is a weight limit of 5 pounds, but other than that, there are no limits as to device physical appearance and functionality. A qualifying round will evaluate consumer experience so user satisfaction is important. Teams are also expected to ensure that consumer safety is held in the highest regard.
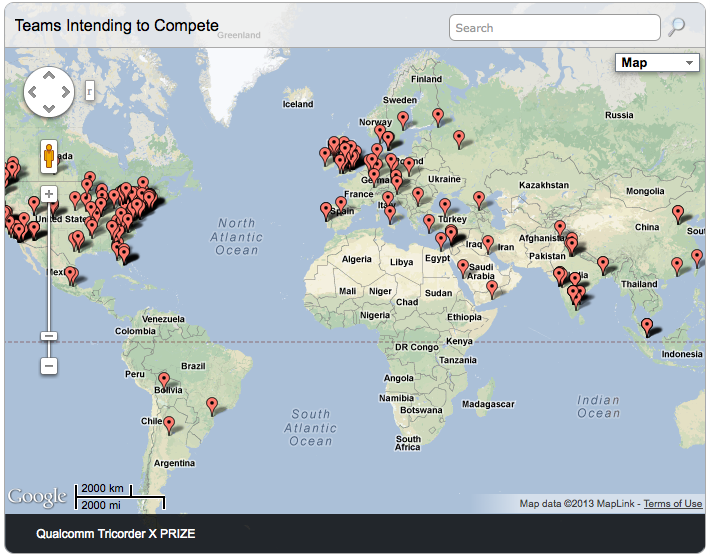
The Qualcomm Tricorder XPrize is truly a global competition. So far, more than 250 teams from around the world have already filed Intent to Compete forms. Look at this map (from the website) to see where the teams come from:
I contacted Qualcomm to get an update on the competition. I interviewed Mark Winter, Senior Director, Qualcomm Tricorder XPrize, to get an update.
To read other posts in this exclusive ongoing series, please visit the Mobile Health Around the Globe main page. And if you have a Mobile Health Around the Globe story to tell, please post a comment below or email me at joan@socialmediatoday.com Thanks!
Video Transcript (by TranscriptionStar)
Joan: Hello I’m Joan Justice from HealthWorks Collective and I’m here today with Mark Winter, Senior Director Qualcomm Tricorder XPrize. The Qualcomm Tricorder XPrize is a 10 million dollar global competition developed to incentivize Health care technology invention. The goal is to develop a handheld device that would allow a consumer to access the state of their own health anytime and anywhere. The device will be able to capture key health metrics and help patients better communicate with their physician. Mark you’re now on the registration process for the Qualcomm Tricorder XPrize and tell us a little bit how things are going. How many teams are there currently?
Mark: We currently have 287 teams that are pre-registered for the competition from 35 countries and we actually expect that to grow in the next few months. And they represent a very interesting mixture of both entrepreneurs and start up ventures and then ventures of all types along with some medium sized companies and even some a larger corporate research and development organizations all who have come together to pursue the vision of this personal consumer medical utility.
Joan: Great yeah I saw on your website 250 so it’s grown since then, now your registration process when it’s over how many teams do you actually think will go into the next phase?
Mark: One key feature of all these competitions that the XPrize foundation puts on we help to facilitate this is some recombination occurs before registration closes and even have those after registration closes. And I think that we’ll probably water down to perhaps over 100 teams but somewhere maybe between 100 and 130 teams. In many cases as these groups get to know one another or we stage events and have the online systems that let them find one another, we see that they start to join forces in order to increase their odds of winning the competition and we expect that to happen as well.
Joan: Great and what’s been the experience for the teams, I bet there must be a lot of networking and mentoring and maybe the possibility of venture capital, it must be exciting for those teams?
Mark: Well I think so; I think one of the things that we’re really trying to do is to bring an entire ecosystem around these teams that will help them succeed. It’s important to know the ultimate end goal of this competition is not simply to reward a 10 million dollar prize, it’s actually a first second and third position, but rather to help all teams that actually compete achieve a level of commercial success and go somewhere with what they’ve developed. So to that degree we’re working closely with the FDA and you actually can see some of the FDA’s videos on our websites but collaborating with them to understand more about how this source of multi functional consumer devices can get through FDA clearance.
Joan: Well.
Mark: ingoing in the future
Joan: Okay.
Mark: We’re talking to large health insurers and CNS to really understand how the insurers might relate to this technology and this is sort of the external market forces and equally how doctors and nurses in large health care systems would perceive and use this device because… and then we expect this is important for them as it is to consumer. And on the team side what we’re really trying to do is to bring together pools of interested people, you mentioned investors, it was exciting to see [Mike Laza Riders] [0:03:38] who is one of the founders of research in motion announcing 100 million dollar fund to fund a medical Tricorder and I personally met with dozens of venture capitalists in the Life Sciences space who are absolutely fascinated with prospect of this type of device and are interested in finding a place to put money. So we’re looking at building an environment that has investors but also has technical resources. We have many different companies which are listed in our online market place system that can provide things like analog to digital conversion techniques and wireless censoring technology that allow a wired censored to become a wireless censor. And other kinds of resources that could help them facilitate the wireless communications capabilities meet things like Hippo which is a data confidentiality requirement for a competition, lots of interested parties have circled their wagons around this competition to help these teams succeed and we’re really doing our best to try to moderate all that and bring that together for the competition.
Joan: Yeah how exciting. Now how do you envision the device, just tell us briefly it’s a consumer product and just tell us briefly what you envision it to be?
Mark: Well you know one way to think about, I’ll give you sort of the span of control the team may have and how they blend this together is entirely up to them. On one hand you’re going to have census of various types that can deduct our primary course date of 12 plus three elective conditions and vital signs that will be communicating in probably many cases wirelessly to the handheld device. So you have a sensor ray which is one block of technology. You have the handheld device itself which generally we think of as a smart phone but it could be a custom embedded device that somebody builds and there have been people working on Tricorders who do build accustomed embedded devices. But likelihood is many people will take advantage of the processing power of smart phones and use them and of course they are communicating through the cloud wirelessly to back in systems which could be electronic health records, data analytic systems, even expert systems like IBM’s Watson which is being readied for healthcare. So how you blend and mix the various capabilities of those three major technology areas into a Tricorder is really up to the team but that’s generally what the span of the competition will look like, sensors, a handheld device and some back end resources.
Joan: And what’s the next step? When do you start the… what you call the qualifying round?
Mark: Well the official registration clause ends in September of this year and then approximately a little less than a year later there is a significant down select to a qualifying group. All of that information is available by the way on the Tricorder website, if you go to xprize. org, and you pull down just price, it’s got a complete schedule and guidelines and other information for anybody that’s interested but they’re probably one of the exciting things for me personally is we’ll be staging in Mid-October the first tem Summit we put together a series of Summits or we bring these teams together, answer the questions about the judging process, we’ve got a fantastic blue ribbon group of judges that they’re aware of and they’ll know about it, they’ll have many questions about the judging criteria, how they technically submit their solutions, they’re all part of this competition and we’ll have a chance to speak to them there. And that is occurring in Mid October and that’s sort of the next set all of that to competition.
Joan: Great well thanks so much Mark for giving us this update, it’s really an exciting competition and I’m sure it’s going to produce some excellent products and it’ll really help the teams and I can’t wait to hear more. Thanks so much.
Mark: Thank you very much I appreciate your time.







