(Editor’s note: Dan Munro writes for Forbes.com under the heading: HealthCare Compass)
(Editor’s note: Dan Munro writes for Forbes.com under the heading: HealthCare Compass)
The pause was brief last Thursday morning as Dr. J. Craig Venter ended his keynote at the CALBIO event in San Diego. It was just long enough for someone to walk up to a floor microphone and ask the question that was absolutely in the air – if not on everyone’s mind. Basically, what did Dr. Venter think of the Supreme Court’s decision on the Myriad Genetics case – which was breaking news that very morning?
“I applaud the Supreme Court’s decision today … which was the right thing to do.” J. Craig Venter, Ph.D., Founder, Chairman and CEO of the J. Craig Venter Institute (JCVI)
Mary-Claire King, the geneticist who actually discovered the BRCA-1 and BRCA-2 genes, was “delighted” and added this quote to an article in Slate.
“Genes had been patented before; the cystic fibrosis gene was patented. But I don’t think anyone – from the U.S. National Institutes of Health or anywhere else – anticipated the level of patent protection Myriad has engaged in.” Mary-Claire King – Geneticist and Professor at the University of Washington
I managed to catch up with Dr. David Agus by email who noted the landmark ruling would transform medicine into a new molecular era.
“Effectively, the Supreme Court has democratized DNA. We all know the decision made sense on moral grounds, but this is a case where it’s so nice to see the judicial system agreeing.” Dr. David Agus – noted author and Professor of Medicine and Engineering at the University of Southern California
The consensus was generally bullish – except of course for Myriad’s stock price (NASDAQ: MYGN). During the breaking news cycle the stock climbed about $5 reaching a peak of almost $38 on Thursday. At Friday’s close, however, it was down to $27.59 – almost 20% down from Wednesday’s close of $33.92.
There were also a number of skeptics inside the biotech industry that seemed to think this landmark ruling was bad for the diagnostics industry generally. I didn’t get a chance to speak with many, but I did manage to connect with Dr. Sherri Bale of GeneRx. Her company plans to release a BRCA test to compete head-to-head with Myriad later this summer. Her view was clearly optimistic.
“We’ve waited a long time for this day, and now the playing field is leveled. We will deliver this test, as well as others, which until now we’ve been restricted from offering. We will now be able to compete on price as well as quality, customer service, and patient-friendly billing policies. Our alternative, called BRACfast, will be available August 1.” Sherri J. Bale, PhD, FACMG, Managing Director, GeneDx and Sr. Vice President, BioReference Laboratories, Inc.
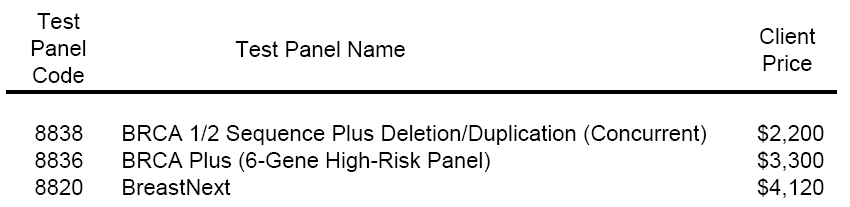
Other genetic testing companies were quick to brandish freshly minted web pages with billing codes and pricing. This chart is a representation of one online PDF available from Ambry Genetics (here):
For a more precise legal interpretation I turned to patent attorney (and DLA Piper Partner) Stacy Taylor. She graciously provided her expert legal opinion as it applies to a broad range of constituents.
Plaintiffs and Patients:
“This is really a pyrrhic victory for the ACLU. I spoke with an ACLU attorney shortly after the initial Myriad complaint was filed. He confirmed the principal intention of the suit was to enable laboratories to offer BRCA testing at substantially lower price points than Myriad and for use in second opinions. By itself, the Supreme Court ruling this week does not necessarily accomplish that end. While testing may be available a few months before Myriad’s patents would have expired anyway, the prices being discussed (still in the thousands) won’t be that much more affordable for patients if insurers don’t cover the test.”
Insurers:
“So far, there doesn’t seem to have been any organized effort to challenge insurers with respect to any diagnostic assay coverage, including BRCA assays. However, once realization sets in that the Myriad decision really didn’t have much impact on assay access, insurers may well be the next target for litigation.
Researchers:
“For future patenting, Myriad merely serves as a roadmap for patent counsel to claim the synthetic DNA counterparts to genomic DNA and methods for its use. Those claims will be more difficult to obtain, but not because of the Myriad decision. Instead, the publication of data from sequencing of the human genome means that gene sequences will be known, so claims to them will be limited. Also, Myriad doesn’t affect claims to methods for using genetic sequences and related data. New diagnostic/prognostic tests will continue to be protectable (subject to limitations in the Mayo v. Prometheus decision). In short, the diagnostics industry is as viable – and fundable – now as it was yesterday.”
Supreme Court Impact:
“Many Court watchers have long felt that the Supreme Court isn’t competent to decide scientifically intensive issues. The Myriad decision doesn’t do anything to change that perception. To the contrary, Justice Scalia essentially admitted in his concurring opinion that he didn’t understand the science well enough to rule on it, though he was willing to accept the majority’s view that synthetic DNA is patent eligible. By itself, that extraordinary admission makes the Mryiad decision worth reading.”
Stacy Taylor – Patent Attorney and DLA Piper Partner (DLA Piper Bio here)
The debate around big research costs and profit incentives remains fairly unaltered – and perhaps unalterable. Nowhere was this more evident than this 4½ min interview clip (here) of Myriad Genetics Founder (and former CEO) Mark Skolnick by Joanna Rudnick for her documentary In The Family. In 2007 she asked the question directly:
Q: “You [Myriad] said this test will one day be 100’s of dollars. Why is it still $3,000? Why is it increasing?” Joanna Rudnick – film documentarian
A: “That’s a good question and I think there’s a point at which we have to start looking at decreasing the cost of the test.” Founder and Former Myriad Genetics CEO – Mark Skolnick
That was 6 years ago. Today the Myriad BRCA-1/2 test is more than $4,000. With the freshly minted Supreme Court ruling we may start to see the mechanics of free market competition bring that cost down – but we’re still a long way from the 100’s of dollars that Joanna Rudnick highlighted back in 2007. Dr. Venter applauded the Supreme Court for doing the right thing. Now it’s time for the biotech and insurance industries to follow that lead.