Each year, sepsis kills far more people than AIDS, prostate cancer, and breast cancer combined. Sepsis is one of the top three causes of death globally, with patients being five times more likely to die from sepsis than heart attacks or strokes. Sepsis is also responsible for 70-90% of the organ failure deaths related to Ebola, yet it goes unrecognized, is poorly understood, and has very low public awareness.
Each year, sepsis kills far more people than AIDS, prostate cancer, and breast cancer combined. Sepsis is one of the top three causes of death globally, with patients being five times more likely to die from sepsis than heart attacks or strokes. Sepsis is also responsible for 70-90% of the organ failure deaths related to Ebola, yet it goes unrecognized, is poorly understood, and has very low public awareness.
Treatment for sepsis often involves a prolonged stay in the ICU, requiring complex therapies costing the US health system over $5,000 per patient/day, or $20 billion/year. Globally, sepsis is estimated to exceed costs of $90 billion/year. Even worse, the mortality rate for sepsis is approximately 35% — higher in developing countries — with patients who survive often struggling with physical impairment, muscle and nerve damage, cognitive changes and chronic organ failure.
According to Patrick Maguire, MD, PhD, MBA and CMO of HemoLife Medical, “Currently the greatest need for fighting sepsis is the ability to manage (reduce) cytokine cascade/storm (hypercytokinemia), and thus prevent the devastating secondary effects of organ compromise and failure. Where we currently have the greatest need is for technologies that can effectively and efficiently decrease cytokine burden. Even if we could detect sepsis earlier, we will always need to effectively treat a run-away cytokine storm in order to help return the patient to a more normal, healthier state.”
The Silent Killer
The inflammatory response is the mechanism our body uses to recognize and defend against an infection. When bacteria, trauma, toxins, heat and other injuries occur to tissue, the body’s automatic response is to fight it. The damaged cells release chemicals that cause blood vessels to leak fluid into the tissues, causing swelling. This helps isolate the foreign substance from further contact with body tissues. However, after a patient’s infection has been controlled, sepsis – or septic shock – can continue in two ways: 1. by the presence of endotoxins (dead bacteria), and 2. cytokines that are released by the body.
In fact, in most cases, the inflammatory response component of sepsis is the true killer because, even after antibiotics have been successful and the patient has been properly hydrated, clinicians can do very little to improve a patient’s odds of surviving.
HemoLife Could Save Lives – And Money
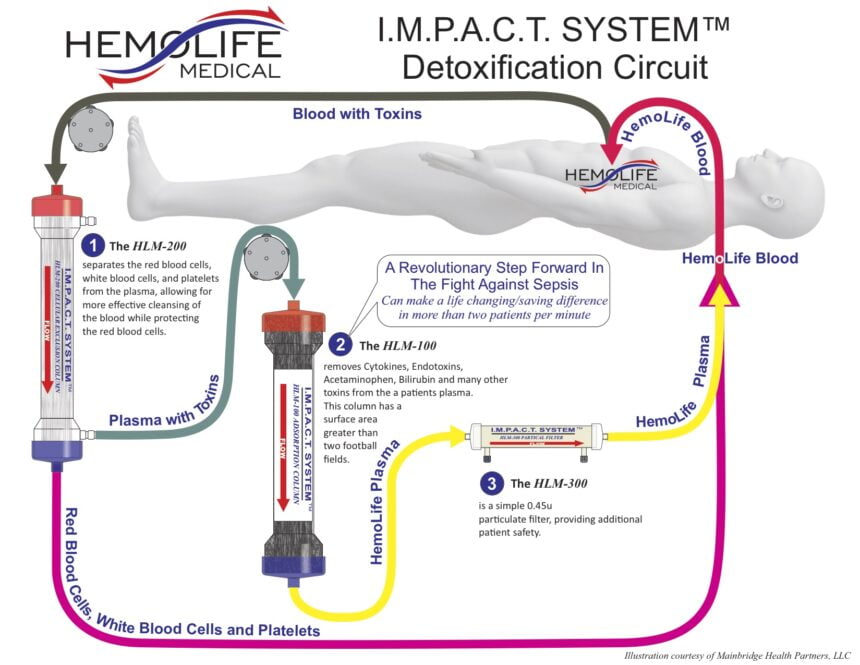
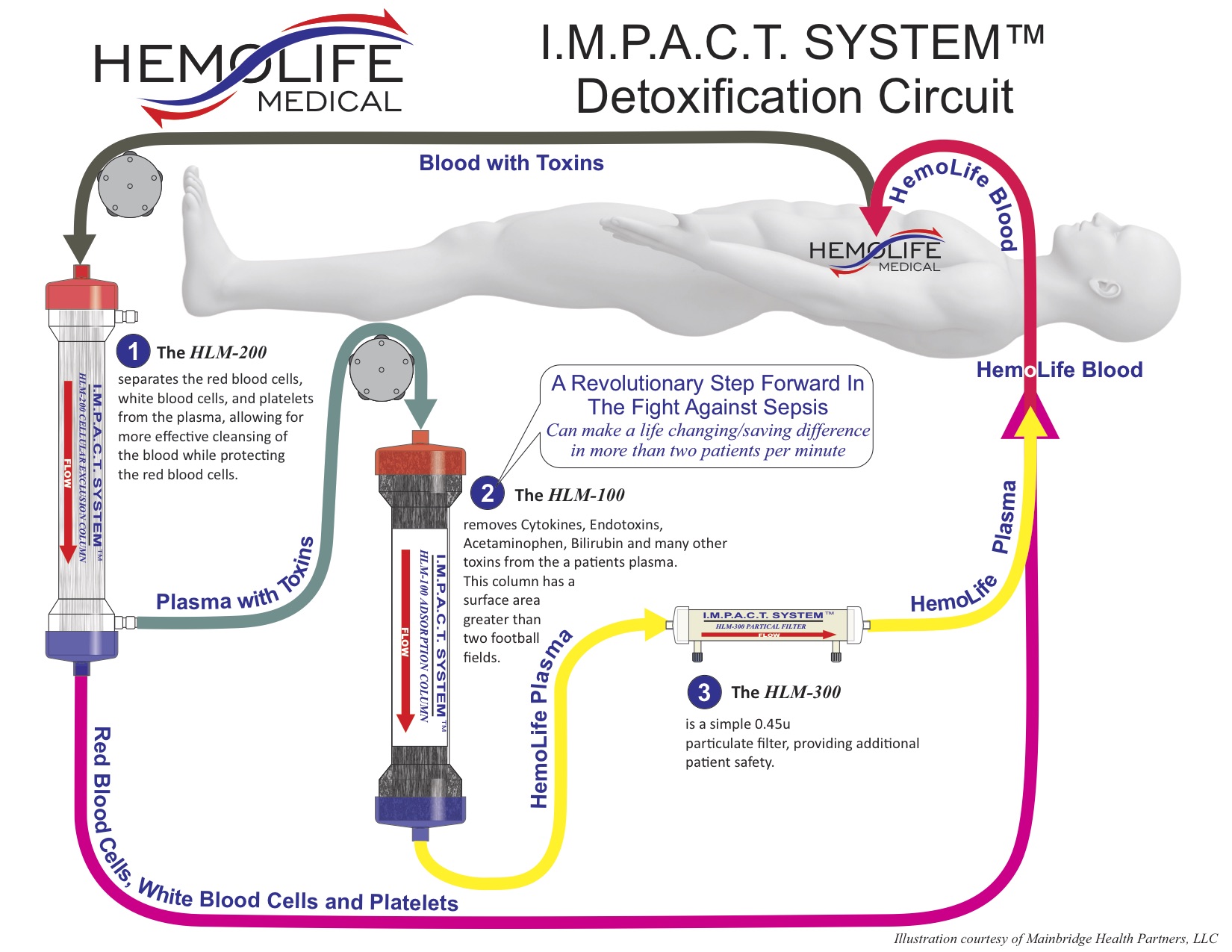
In addition to improved diagnosis and awareness, new products are entering the market that could forever alter the way sepsis is treated, first and foremost by simply providing a more direct “treatment”. One such proposed treatment entering the market that filters toxins from the blood stream is the IMPACT System by HemoLife. The company, HemoLife Medical, believes that by effectively removing cytokines and endotoxins from a patient’s blood stream, their system reduces the primary triggers driving a patient’s destructive inflammatory response that occurs during sepsis. In most cases, patients can be effectively treated with as few as three HemoLife treatment cycles.
The IMPACT ‘HemoFilter’ minimizes electrolyte and protein depletion from the treated plasma, effectively removing both free and protein-bound toxins. The toxin removal device works by separating red blood cells, white blood cells, and platelets from the plasma, followed by filtering out specified toxins from the plasma. After filtration is complete, ‘clean blood’ is infused back into the body, reducing the toxins that fuel the life threatening inflammatory response called sepsis.
Although the clinical success of the IMPACT System has been shown and approved for sale in the European Union (EU), Canada, India and Australia, “FDA approval is expected in the US during the fourth quarter of 2016,” says Mike Ward, President of Mainbridge Health Partners, the company taking HemoLife to market. Mainbridge’s goal with HemoLife is to “improve health care providers’ understanding of the IMPACT System effectiveness on sepsis, so we can more rapidly make it standard of care as it enters the US market. Our investors are helping us make that happen right now, so we can start saving lives both domestically and abroad.”
The IMPACT Could Be Global
According to Lon Stone, President and CEO of HemoLife, “The IMPACT System, as an adjunct treatment is currently distributed to several international hospitals, has the potential to improve patient survival and reduce the length of stay in ICUs, resulting in cost savings from $5,000-$10,000 per day.”
The statistics showing the devastation of sepsis in these countries are staggering. Approximately every three seconds a patient dies of sepsis, and more than 6 million of these deaths every year occur in children. In those locations sepsis is also the second most common case of maternal death – second only to bleeding.
Just like the US and other developed nations, developing countries could use products like HemoLife’s for cost savings and life savings. The IMPACT System would need to access equipment that is presently not available in some parts of the world, but the utilization and training for the product could potentially become universal as hospitals, clinics and mobile facilities grow around the world.
Dr. Maguire believes, “As more providers, clinics and traditional hospitals become comfortable with the IMPACT System’s safety profile and results, the opportunity to move this treatment earlier in the patient’s clinical course should enhance patient outcomes, further reducing morbidity and mortality. The possibility to intervene in the treatment of graft versus host disease (GVHD), acute pancreatitis, acute respiratory distress syndrome (ARDS), avian influenza, and systemic inflammatory response syndrome (SIRS) remain applications to be explored, and the ones for which we have the greatest hope.”