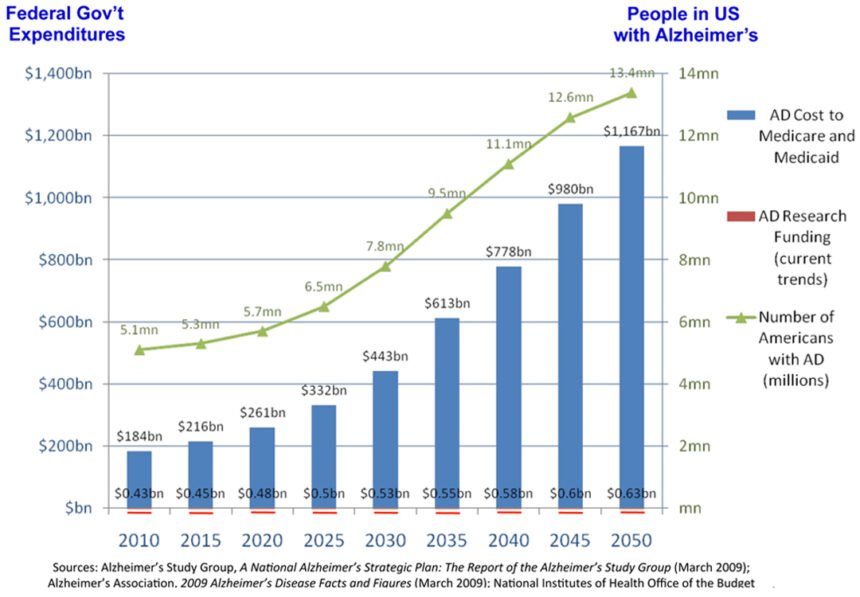
Alzheimer’s disease is the most expensive condition in the nation. The estimated cost of care for Alzheimer’s disease in the US alone exceeded $214 billion in 2014, with nearly one in every five dollars spent by Medicare on dementia. Further, future cost estimates from the Alzheimer’s Association predict that by 2050 the disease will cost $1.2 trillion annually. The disease currently affects five million people in the US, and expected to grow to 16 million by 2050, afflicting one in nine people over the age 65, and one in three people over the age of 85. With patients, care providers, researchers and policymakers desperate for a definitive test, the world is one step closer to having results.
But would you want to know if you are going to develop – or are already in pre-symptomatic early stages of – Alzheimer’s disease? When there is currently no known cure and only marginal progress in symptomatic treatments, what good or harm comes from knowing? This hypothetical is one step closer to being your reality. Last week, Congressman Chaka Fattah (D-PA), architect of the Fattah Neuroscience Initiative, met with Amarantus Bioscience Holdings, a biotech company that is developing a set of neurology-focused diagnostic products, to discuss the importance of genomic testing for Alzheimer’s disease, the growing need of more federal funding for Alzheimer’s research and the company’s new Lymphocyte Proliferation Test (LymPro Test®) blood diagnostic test that it claims uses biomarkers to identify at-risk individuals for Alzheimer’s.
According to Dr. Howard Federoff, Executive Vice President for Health Sciences and Executive Dean of Georgetown University Medical Center, he is “exited there is a means by which we can test people. The excitement falls on the fact that we have failed many times in the past and spent a lot of money, as has everyone in this field. Now we can test preclinically as to enable people to take preventative measures or in the future cure it. Additionally, because a preexisting condition no longer means that you can be precluded from long-term care and life insurance, financial planning is now possible for individuals, families, employers and societies.”
The dichotomy of those who want to know and those who do not is separated by a deep chasm in beliefs, and could forever alter the way individuals, families and wealth managers prepare for disease, as well as how insurers and care providers pay for and provide care. While genetic tests are presently able to determine about 4,000 diseases and disorders, the breakthrough of predicting Alzheimer’s disease could vastly change the behaviors and costs to society, actually bending the cost curve health experts so often talk about as necessary for economic stability. Until now, official diagnosis of the disease has been reserved for the autopsy table, an unacceptable situation.
In January, Amarantus reported positive top-line results of its LymPro Test®blood diagnostic for Alzheimer’s disease. The Company also entered into an exclusive option agreement with Georgetown University (GU) to commercialize sets of blood-based biomarkers for Alzheimer’s disease. The Company has said it is positioning itself to capture the lion’s share of the Alzheimer’s blood test market – eventually worth an estimated $3 billion.
But how will individuals react? How about the care provider and insurance markets? When there is presently no cure, it is expected that individuals and families will be torn on the decision to test. In contrast, it seems inevitable that employers, health officials and insurers will want to know the future demands, needs and composition of their populations.
A Game-Changing Blood Test
Blood-based biomarkers of preclinical disease will certainly be critical for preventative or disease modifying advances in the coming years. However, to date, these biomarkers for early disease detection have not been accurate, are extremely expensive, time consuming and invasive. However, Dr. Federoff claims that their lipid approach has revealed, “ten lipids from peripheral blood that predicted phenoconversion to either amnestic mild cognitive impairment or Alzheimer’s disease within a 2–3 year timeframe with over 90% accuracy. More exciting, we currently have, under review, the metabolomics data that could go even further, showing 99% accuracy measuring 17 lipids.”
The group’s exosome work was recently published in an Alzheimer’s Association’s publication providing a second important modality to GU’s conceptual understanding of the disease. According to Dr. Federoff, this “secondary prevention is a whole new kind of clinical trial,” that cannot be ignored much longer. Congressman Fattah, who has led Congress in efforts to increase public funding for brain research was also named as one of four co-chairs of the House Alzheimer’s Disease Caucus last week, has also committed to using information like this to, “advocate for policies, research, and funding that will advance our efforts to fight Alzheimer’s disease.”
While LymPro® is not ready for the shelves of CVS or Walgreen’s, it may not be long before in clinic and in home testing is available to consumers. Without a cure or highly effective symptomatic treatments to go hand-in-hand with the blood test, the risk and reward of such a test will certainly take a toll on individuals, families, health care markets and communities.
Amarantus says it will publish data from its studies in peer-reviewed journals as well as present them at various scientific conferences throughout 2015.