Gene therapy can have tremendous benefits for people struggling with serious health problems around the world. The National Institute for Health reports that future gene therapy treatments can help with a number of inherited illnesses, including hemophilia, sickle cell disease and cystic fibrosis.
We have talked about the latest gene therapy research and the benefits they can have for our health. Many of the recent breakthroughs in gene therapy are derived from gene targeting advances in mice.
Gene Targeting in Mice Can Be Invaluable for Humans Struggling with Genetic Illnesses
Gene targeting is a homologous recombination-based alternative strategy technique used to modify endogenous genes, which can be implemented for gene deletion, addition, replacement, and base-pair modification. The gene-targeting technique has developed over the decades. It is incredibly interesting to understand when we move from the discovery of the double helix (Watson and Crick, 1953) to the discovery of restriction endonuclease (Werner Arber, 1968) and recombinant DNA technology (Stanley N. Cohen and Herbert W. Boyer, 1972) and the development of most advanced gene-editing tool CISPR-Cas (Jennifer Doudna, Emmanuelle Charpentier, and co-workers, 2012).
There are a number of compelling reasons to invest in gene targeting research. They can help with tissue engineering and cell therapy, which may cure many health problems.
However, medical scientists still need to make massive progressive in gene targeting before health experts can apply this research. Fortunately, there are some major advances on the horizon. Many of them are due to research on mice, which may be the key to solving many health problems in humans with genetically inherited diseases.

Fundamental and technical issues of traditional gene targeting
The fundamental issue with gene targeting is the existence of genetic compensation response. Researchers sometimes find that even though the target gene has been identified and verified to be completely silenced, the organism does not lack a functional gene product, but can be detected with corresponding phenotypes as if the gene was not functional. An explanation for this phenomenon is that the gene must be compensated by “helper” genes, as the analysis suggests.
Gene targeting is accomplished following the transfer of genes, and the most difficult step is the transfer of genes in stem cells. It can be addressed using a highly specific vector with high efficiency for required size gene release, non-detectable by the immune system, easy purification, and large quantity for large-scale application. Following insertion, there may be inflammation or allergic reactions; it should correct the deficiency, hinder harmful activities, and augment the normal function. Introduction new gene inside a cell and keeping that working is highly specific.
Flanking allele or hitchhiking gene issue: Alterations occur in an allele not because of natural selection but rather due to nearby genes present on the same chromosomes. Such issues develop due to the insertion of a new gene in genetic material.
Genetic variance-related issues: Alterations in the sequence of a gene are genetic variances, due to which malfunctioning of proteins translated from that gene occurs.
The recent advancement in gene targeting techniques
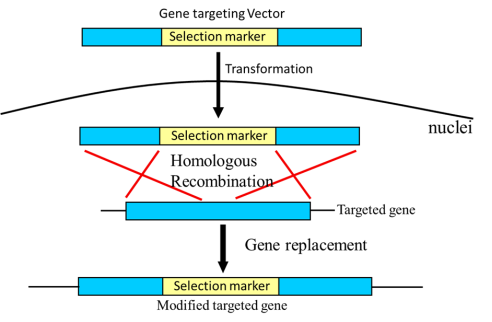
1. Homologous recombination-based gene targeting
In gene targeting by homologous recombination, the exchange of genetic material between the genome and exogenous deoxyribonucleic acid occurs by cross-over. Homologous sequence acts via cellular enzymatic machinery that guides these exchanges. In the gene of interest, targeted alterations are accomplished through homologous recombination. Such modification mechanisms facilitate the exploration of gene function (functional genomics) and the human genetic diseases model. So this makes gene targeting technology a valuable gizmo for modern genomic studies and related disease treatment. Technology advancement allows direct enzyme repair by inducing target site-specific DNA damage. It is achieved by using transcription like activator effector nucleases, zinc-finger nucleases, triplex-forming oligonucleotides, and CRISPR (clustered regularly interspaced short palindromic repeats). The ribonucleic acid interface (RNAi) helps to ensure the suppression of anti-recombinogenic pathways. Such techniques revolutionized gene targeting efficiency.
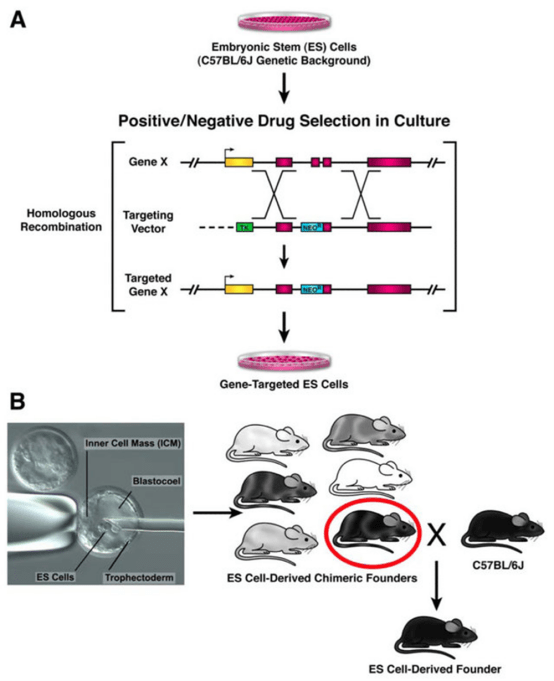
2. ES Cell-Based Gene Targeting
In embryonic stem cells, the endogenous gene locus is altered via homologous recombination, which will help generate mice with targeted mutations. Embryonic stem cells carrying the desired gene mutation are microinjected into blastocyst stage embryos. These microinjected embryos will then be transferred into pseudopregnant surrogate mother mice for further development. These surrogate mice will deliver and nurse the chimeric offspring, which are then bred to establish the knockout mouse line.
Applications and advantages for the healthcare sector regarding the two technical advancements.
There are a number of ways that the healthcare sector can benefit from this research:
- For determining the gene function through insertion or deletion mutation
- Animal model development (via insertion of the human genome in animals) for exploring the relationship between human diseases and genes. (Mainly model is used due to susceptibility of mouse model towards human pathogens)
- The pathogen gene is inserted for evaluation of such gene in the pathogenicity of a specific pathogen (Mainly viruses)
- The reporter gene is introduced for in vivo as well as in vitro gene expression analysis.
Cyagen, a well-reputed company, provides genetically engineered mice as well as services for gene editing. The targeting mutations in the specific cell or tissue or whole mouse gnome can help develop the transgenic and knockout mouse. A period of about 1 year is needed for the development of the gene-targeted mouse.